SAVE SOLUTIONS
Cathodic
Protection
Deep Knowledge in Cathodic Protection Systems Design
SAVE Engineering carries out accurate analysis processes, which is the key to efficient project design and offers long-lasting solutions with the correct determination of all elements in system design.

Permanent Protection
The decisions taken during the analysis and projecting stages of cathodic protection systems affect the efficiency and life of the system. SAVE Engineering aims to provide long-lasting, sustainable protection during the system design process.

How Does Metal Corrode?
Corrosion occurs as a result of the anode and cathode reactions occurring on the metal surface together.
The 3 factors required for these reactions are as follows:
1. Anode and cathode (Metal with two different potentials)
2. Electrolyte (saline solution)
3. Electrical connection between anode and cathode
The term metal at two different potentials usually refers to two identical metals with microscopic and macroscopic metallurgical differences, as well as completely different alloys such as steel and aluminum.
For example, the anode cathode reactions on the iron surface are as follows:
Anode reaction: Fe → Fe 2+ + 2 e-
Cathode reaction: ½ O2 + H2O + 2e- → 2OH-
In anode and cathode reactions, electrons flow from the anode to the cathode through the junction metal. Cathode reactions take place using these incoming electrons. If we prevent the use of electrons at the cathodes, that is, if we keep the oxygen away from there, we prevent corrosion.
How Does Cathodic Protection Stop Corrosion?
Cathodic protection is an effective protection method that definitely prevents corrosion of metals. The method is based on the theory of electrochemical corrosion. And it can be explained as removing the anode currents on the metal surface by making the metal to be protected as the cathode of the electrochemical cell. There are two types of protection systems that can achieve this:
1. Galvanic Anode Cathodic Protection Systems
2. Impressed Current Source Cathodic Protection Systems
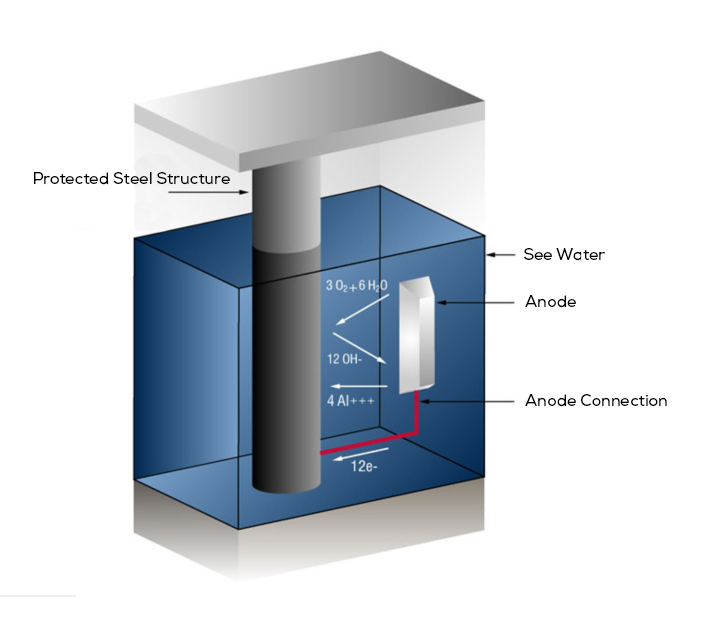
1. Galvanic Anode Cathodic Protection Systems:
Galvanic Anode Method, also called Sacrificial Anode Method, is a system that works with the principle of sacrificing another metal instead of corroding the metal we want to protect.
Galvanic Anode Method, also called Sacrificial Anode Method, is a system that works with the principle of sacrificing another metal instead of corroding the metal we want to protect. In this system, another metal with a more negative potential is attached to the metal to be protected from corrosion. In this way, the electrons required for the cathode reactions are obtained from the galvanic anode. These anodes are preferred from alloys such as zinc, aluminum and magnesium.
When designing the cathodic protection system, the anodes must be sized to have the correct shape and surface area to drain sufficient current. And while discharging this current, galvanic anodes that will sustain the desired life and have sufficient weight should be used.
The effectiveness of the protection system can be increased by covering the metal surface to be protected with a suitable material in order to reduce the current requirement.

2. Impressed Current Source Cathodic Protection Systems
Impressed current sourced cathodic protection is applied to the metal by giving an external direct current. The alternating current taken from the network is converted into direct current obtained from a transformer rectifier system. The negative end is connected to the metal to be protected, and the positive end is connected to an auxiliary anode. Various metals that can give the desired current without the need for high potential are used as auxiliary anodes. At the same time, they must be able to last for a long time without breaking down.
Although the initial investment costs are lower than the galvanic anode system, the operating costs are high. If the electrolyte resistance is high, the DC potential can be adjusted in the transformer unit. This process can be carried out manually as well as automatically by placing a fixed reference electrode in the cathodic protection circuit.
Save Solutions
Why SAVE Engineering
in Cathodic Protection?
Cathodic Protection in Marine Structures
In the field of designing and implementing cathodic protection systems in marine structures, SAVE Mühendislik provides services with theYNS Corrosion brand, which is a consortium formed with Norse and Yeke companies.

Sign up for our newsletter
Subscribe to receive SAVE Mühendislik's newsletters and be informed about new products and projects.
